Stryker Accolade Lawsuit
(Hip Implant)
Problem summary
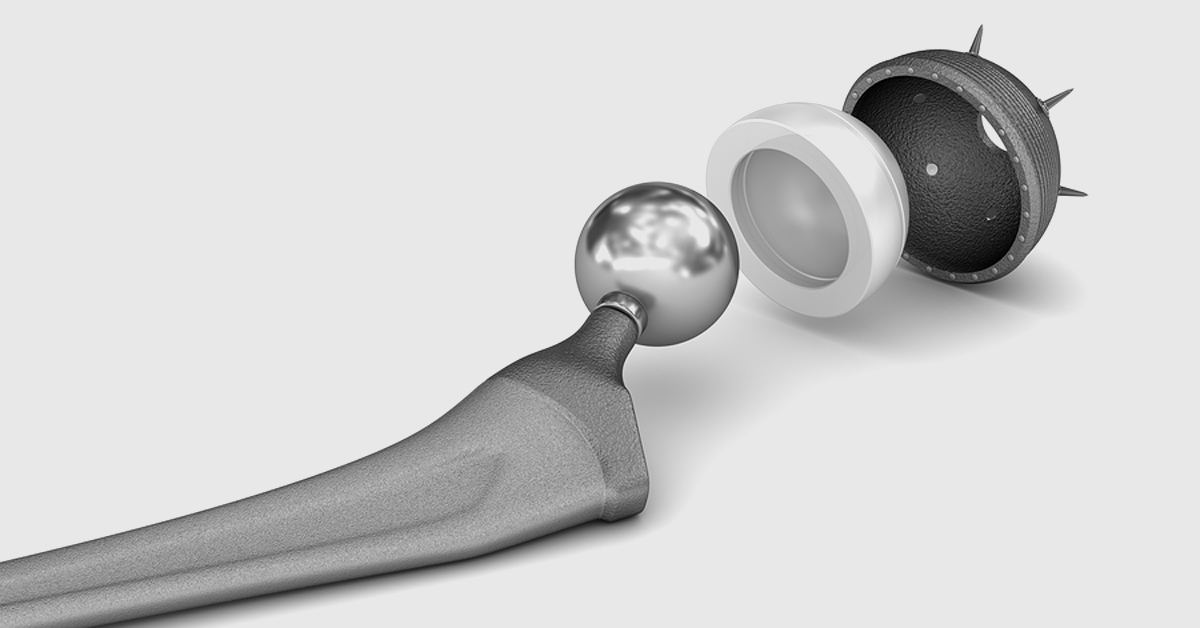
The Accolade hip replacement system uses several neck and stem components that are meant to be interchangeable, to help surgeons provide custom-fit designs for patients requiring hip arthroplasty, compared to traditional implants, which typically use one larger piece. Despite the perceived benefits of this type of system, research has shown that Accolade stems, used in combination with LFIT V40 femoral heads and other metal components, may be prone to premature failure due to fretting and corrosion at the head/stem connection. And while it was initially believed that this early failure occurred only with larger heads, a growing body of evidence shows that smaller heads may also corrode and snap within just a few years after the initial hip replacement surgery, possibly causing catastrophic hip failure or spontaneous dislocation. Many patients implanted with Stryker’s Accolade and LFIT V40 hip systems have experienced debilitating pain as a result of premature failure and have been forced to undergo a second surgery to replace the faulty prosthetic hip.
About Stryker Accolade Hips
The Accolade TMZF hip stem is a hip replacement component made from one single piece of titanium alloy and the device features a tapered wedge designed to accommodate more surgical approaches and to fit a greater number of patients. The Accolade hip implant is manufactured by Stryker Orthopedics and was approved by the FDA in 2000, via the agency’s fast-track 510(k) approval process. At that time, Stryker, like many metal-on-metal hip implant manufacturers, claimed that the all-metal construction of its Accolade device made it more durable than earlier-generation implants and provided better range of motion. However, much like Stryker’s Rejuvenate and ABG II hip implants, it was quickly discovered that the Accolade implant put recipients at risk for dislocation, metallosis and other complications requiring painful revision surgery.
Problems with the Accolade hip stem caused Stryker to issue several device recalls of various hip implant models, in 2009, 2011 and 2013. Stryker’s Rejuvenate and ABG II hip implants were also recalled in 2012, due to a higher-than-expected rate of early failure, and the same combination of mixed metals exists when a Stryker Accolade hip stem is used with a metal acetabular cup. Metal-on-metal hip implants such as this have come under fire recently for debilitating complications allegedly occurring when the metal hip components rub against one another during use, which causes tiny metal particles to be shed into the bloodstream. Metallosis, a condition characterized by high levels of metal ions on the bloodstream, is a type of metal toxicity that can cause dangerous systemic reactions in other parts of the body.
Stryker Accolade hip stems are often used with Stryker’s LFIT V40 femoral heads for a total hip replacement, also known as hip arthroplasty, and both of these components have been plagued by reports of serious complications requiring revision surgery. In 2016, Stryker recalled more than 42,000 LFIT V40 femoral heads for taper lock failure, an issue affecting the part of the device that connects the cobalt-chromium femoral heads to the hip stem, and one that can cause spontaneous dislocations, a build-up of metal debris in body tissue and other problems requiring additional surgeries. According to one study published in The Journal of Arthroplasty in 2017, involving 28 patients with either trunnion failure or head-neck taper corrosion of the LFIT femoral head and Accolade stems, all of the patients suffered adverse local tissue reactions resulting in the need for revision surgery.
Free Confidential Case Review
Stryker Accolade Hip Injuries
- Persistent pain
- Restricted movement
- Metal blood poisoning (metallosis)
- Fluid collections
- Pseudotumors
- Dissociation of femoral head from hip stem
- Bone loss
- Tissue damage
- Implant failure
- Device dislocation
- Excessive wear
- Nerve damage
- Clicking or grinding sound with use
- Adverse local tissue reactions
- Trunnionosis
- Need for revision surgery
Call Now for a Free Stryker Accolade Case Review
Stryker Accolade Hip Resources and Studies
Stryker Accolade Hip Settlements & Litigation
Hundreds of thousands of patients across the country with damaged hip joints caused by severe arthritis or traumatic injury underwent hip replacement surgeries with metal-on-metal hip implants like Stryker’s Accolade device, amid promises that the procedure would increase their mobility and reduce their pain. Due to severe complications resulting from these hip replacements, product liability lawsuits have been brought against Stryker Orthopedics (Howmedica Osteonics) by recipients of faulty implants. These claims accuse the company of failing to provide adequate warnings about the potential for the Accolade hip implant to expose recipients to higher rates of corrosion and metal toxicity when used with the LFIT V40 femoral head and other metal components. Lawsuits over Stryker’s recalled Rejuvenate and ABG II hips resulted in settlements totaling $2 billion, and patients implanted with Accolade hip stems are now seeking financial compensation from Stryker for the cost of revision surgery, past and future medical bills, pain and suffering, lost wages, loss of future earning capacity, permanent disability, and other related damages.
Free Case Review
Members of our legal team are available 24 hours a day, 7 days a week. If you or a loved one would like a free evaluation of your potential case, please give us a call or complete our no obligation case review form.